1 4 Di T Butyl 2 5 Dimethoxybenzene
shadesofgreen
Nov 06, 2025 · 10 min read
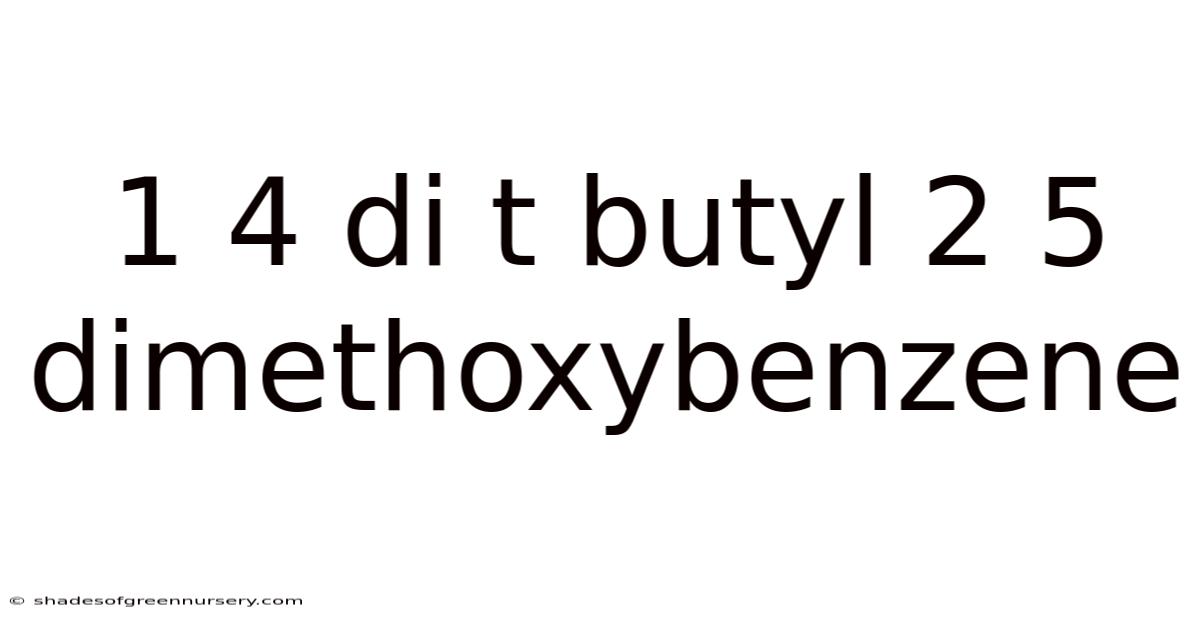
Table of Contents
Okay, here's a comprehensive article about 1,4-di-t-butyl-2,5-dimethoxybenzene, designed to be SEO-friendly, informative, and engaging.
1,4-Di-t-butyl-2,5-dimethoxybenzene: A Deep Dive into Synthesis, Properties, and Applications
Organic chemistry, with its vast landscape of molecules and reactions, often yields compounds with unique properties and potential applications. Among these, 1,4-di-t-butyl-2,5-dimethoxybenzene stands out as an interesting molecule. Its structure, a benzene ring adorned with bulky tert-butyl groups and electron-donating methoxy groups, imparts distinct characteristics that influence its reactivity and uses in various fields. This article aims to provide a comprehensive overview of this compound, exploring its synthesis, properties, applications, and some of the latest research associated with it.
The beauty of organic chemistry often lies not just in the final product but in the journey of its creation. Consider the synthesis of a complex molecule as akin to composing a symphony. Each step, each reagent, and each reaction condition plays a crucial role in creating the final masterpiece. 1,4-di-t-butyl-2,5-dimethoxybenzene is one such "masterpiece" that requires careful orchestration to synthesize. Understanding its synthesis is vital not only for chemists who wish to create it in the lab but also for anyone interested in appreciating the elegance and precision of organic synthesis.
Synthesis of 1,4-Di-t-butyl-2,5-dimethoxybenzene
The synthesis of 1,4-di-t-butyl-2,5-dimethoxybenzene generally involves multiple steps, starting from a simple benzene derivative and building up the desired structure. There are several synthetic routes available, but a common approach involves the following steps:
-
Introduction of the tert-butyl groups: This is often achieved through a Friedel-Crafts alkylation reaction. Benzene or a substituted benzene is reacted with tert-butyl chloride or tert-butyl alcohol in the presence of a Lewis acid catalyst, such as aluminum chloride (AlCl3) or iron(III) chloride (FeCl3). The tert-butyl group is bulky, which can influence the regioselectivity of the reaction, favoring substitution at the 1 and 4 positions, although mixtures of products may be formed, requiring separation techniques like chromatography.
-
Introduction of the Methoxy Groups: Once the tert-butyl groups are in place, the next step is to introduce the methoxy groups. This usually involves two key transformations:
- Hydroxylation: Introducing hydroxyl (-OH) groups at the 2 and 5 positions. This can be accomplished through various methods, including electrophilic aromatic substitution with a suitable electrophile followed by reduction or through more modern cross-coupling reactions.
- Methylation: Once the hydroxyl groups are in place, they are methylated to form the methoxy groups (-OCH3). This is typically done using a methylating agent such as dimethyl sulfate (DMS) or methyl iodide (MeI) in the presence of a base.
A Detailed Example Synthesis
While specific reaction conditions vary depending on the starting materials and reagents used, here's a more detailed, illustrative synthesis:
-
Di-tert-butylation of Benzene: Benzene is reacted with tert-butyl chloride and aluminum chloride in a suitable solvent (e.g., dichloromethane). The reaction is carefully monitored to control the degree of alkylation and minimize the formation of tri- or tetra-tert-butylated products. The resulting mixture is separated, and the 1,4-di-tert-butylbenzene is isolated.
-
Hydroxylation: The 1,4-di-tert-butylbenzene is then subjected to a hydroxylation reaction. One approach involves converting the benzene ring into a quinone derivative (e.g., using an oxidizing agent). The quinone can then be reduced to a diol (i.e., 1,4-di-tert-butylbenzene-2,5-diol).
-
Methylation: The diol is then treated with dimethyl sulfate and a base (e.g., potassium carbonate) in a suitable solvent (e.g., acetone). The methoxy groups are installed, yielding the final product, 1,4-di-tert-butyl-2,5-dimethoxybenzene.
-
Purification: The final product is purified using techniques such as column chromatography or recrystallization to ensure high purity.
Important Considerations in Synthesis
- Regioselectivity: Directing the substituents to the desired positions on the benzene ring can be challenging. The bulky tert-butyl groups influence the regioselectivity, but careful selection of reaction conditions and protecting group strategies may be required to achieve the desired substitution pattern.
- Yield: The overall yield of the synthesis can be affected by various factors, including the efficiency of each step, the formation of byproducts, and the losses during purification. Optimization of reaction conditions is crucial to maximize the yield.
- Safety: Some of the reagents used in the synthesis, such as dimethyl sulfate and aluminum chloride, are hazardous and require careful handling. Appropriate safety precautions should be taken when performing these reactions.
Properties of 1,4-Di-t-butyl-2,5-dimethoxybenzene
The unique structure of 1,4-di-t-butyl-2,5-dimethoxybenzene dictates its physical and chemical properties. The presence of bulky tert-butyl groups and electron-donating methoxy groups significantly influences its behavior.
-
Physical Properties: At room temperature, 1,4-di-t-butyl-2,5-dimethoxybenzene is typically a crystalline solid. Its melting point is influenced by the strength of the intermolecular forces, which are affected by the bulky tert-butyl groups that hinder close packing of the molecules. It is soluble in many common organic solvents, such as chloroform, dichloromethane, and toluene, but its solubility in water is very low due to its hydrophobic nature.
-
Electronic Properties: The methoxy groups are electron-donating substituents, increasing the electron density of the benzene ring. This makes the ring more susceptible to electrophilic attack. The tert-butyl groups, while being alkyl groups, also have a slight electron-donating effect. The combination of these effects influences the molecule's redox potential and its ability to participate in electron transfer reactions.
-
Steric Properties: The bulky tert-butyl groups create significant steric hindrance around the benzene ring. This steric bulk can prevent reactions from occurring at positions adjacent to the tert-butyl groups and can influence the conformation of any substituents attached to the ring. This steric protection can also enhance the stability of the molecule and any reactive intermediates formed from it.
-
Chemical Properties: Due to the electron-donating nature of the methoxy groups, the benzene ring is activated towards electrophilic aromatic substitution. However, the tert-butyl groups provide steric hindrance, which can affect the regioselectivity of these reactions. The molecule can also undergo reactions involving the methoxy groups, such as cleavage by strong acids or bases. The stability conferred by the tert-butyl groups can also make the molecule resistant to certain types of degradation.
Applications of 1,4-Di-t-butyl-2,5-dimethoxybenzene
The unique properties of 1,4-di-t-butyl-2,5-dimethoxybenzene make it useful in various applications, primarily in organic synthesis, materials science, and as a ligand in coordination chemistry.
-
Organic Synthesis: This compound can serve as a building block or a protecting group in organic synthesis. The tert-butyl groups can be used as temporary blocking groups to direct reactions to specific positions on the benzene ring. The methoxy groups can be modified to introduce other functional groups, allowing for the synthesis of complex molecules.
-
Materials Science: 1,4-Di-t-butyl-2,5-dimethoxybenzene can be incorporated into polymers or used as a component in organic electronic materials. Its redox properties and ability to undergo reversible oxidation and reduction make it useful in organic batteries and other energy storage devices. The bulky tert-butyl groups can also prevent aggregation of the molecules, improving the performance and stability of the materials.
-
Ligand in Coordination Chemistry: The methoxy groups can coordinate to metal ions, making 1,4-di-t-butyl-2,5-dimethoxybenzene a potential ligand in coordination chemistry. The bulky tert-butyl groups can create a sterically hindered environment around the metal center, which can influence the reactivity and selectivity of catalytic reactions.
-
Redox Shuttles: Due to its redox properties, 1,4-di-t-butyl-2,5-dimethoxybenzene can be used as a redox shuttle in various electrochemical applications. It can mediate electron transfer between different redox-active species, improving the efficiency of electrochemical processes.
Recent Research and Developments
Research involving 1,4-di-t-butyl-2,5-dimethoxybenzene continues to evolve, with recent studies exploring its use in:
- Organic Electronics: Researchers are investigating the use of this compound as a component in organic light-emitting diodes (OLEDs) and organic solar cells. Its electronic properties and ability to self-assemble into ordered structures make it a promising candidate for these applications.
- Catalysis: The molecule has been explored as a ligand in various catalytic reactions. The steric bulk of the tert-butyl groups can influence the selectivity of the reactions, allowing for the synthesis of specific products.
- Supramolecular Chemistry: Studies are investigating the use of this compound as a building block in supramolecular assemblies. The bulky tert-butyl groups can promote the formation of specific structures, such as cages or channels, which can be used for molecular recognition or encapsulation.
Tips and Expert Advice for Handling and Utilizing 1,4-Di-t-butyl-2,5-dimethoxybenzene
As an experienced chemist, here are some tips and expert advice to consider when working with 1,4-di-t-butyl-2,5-dimethoxybenzene:
-
Purity is Key: Ensure the compound is of high purity before use. Impurities can significantly affect its properties and reactivity. Recrystallization or column chromatography are effective methods for purification.
-
Solubility Considerations: When using this compound in reactions or materials, carefully consider its solubility. Choose a solvent that dissolves it well but does not interfere with the desired chemistry.
-
Steric Effects: Be mindful of the steric hindrance caused by the tert-butyl groups. This can affect the rate and selectivity of reactions. Consider using smaller reagents or catalysts to overcome this steric barrier.
-
Redox Properties: If utilizing its redox properties, ensure the appropriate electrochemical conditions are maintained. Control the pH, potential, and electrolyte to optimize the electron transfer processes.
-
Storage: Store the compound in a cool, dry place away from light and air to prevent degradation. An inert atmosphere, such as nitrogen or argon, can help extend its shelf life.
FAQ: Frequently Asked Questions
Q: Is 1,4-di-t-butyl-2,5-dimethoxybenzene commercially available? A: Yes, it can be purchased from chemical suppliers. However, it might be more cost-effective to synthesize it if large quantities are needed.
Q: What are the primary hazards associated with this compound? A: While not acutely toxic, it's essential to handle it with standard laboratory precautions. Avoid skin and eye contact, and use it in a well-ventilated area. Refer to the Material Safety Data Sheet (MSDS) for detailed safety information.
Q: Can the tert-butyl groups be removed after use as protecting groups? A: Yes, under specific conditions, the tert-butyl groups can be cleaved, typically using strong acids.
Q: What are the alternatives to synthesizing this compound? A: Depending on the desired application, there might be alternative compounds with similar properties. However, the unique combination of steric hindrance and electronic properties offered by 1,4-di-t-butyl-2,5-dimethoxybenzene is often irreplaceable.
Q: How can I characterize the synthesized compound to confirm its identity and purity? A: Common characterization techniques include NMR spectroscopy (1H and 13C), mass spectrometry, melting point determination, and elemental analysis.
Conclusion
1,4-Di-t-butyl-2,5-dimethoxybenzene is a fascinating molecule with a unique combination of properties stemming from its structure. Its synthesis, although multi-step, allows chemists to create a compound with specific steric and electronic characteristics. These characteristics make it valuable in various applications, from organic synthesis and materials science to coordination chemistry and redox shuttles. Ongoing research continues to explore new uses for this compound, highlighting its versatility and potential.
How do you envision using 1,4-di-t-butyl-2,5-dimethoxybenzene in your research or applications? What other compounds with similar structural features and properties intrigue you?
Latest Posts
Latest Posts
-
How Do Opioid Receptors Prevent Substance P
Nov 06, 2025
-
Can You Take Melatonin Before Surgery
Nov 06, 2025
-
Cardiovascular Complications Of Diabetic Kidney Disease
Nov 06, 2025
-
Officials Depend On Peer Pressure For Covid 19 Compliance
Nov 06, 2025
-
Tfam Overexpression Reduces Pathological Cardiac Remodeling
Nov 06, 2025
Related Post
Thank you for visiting our website which covers about 1 4 Di T Butyl 2 5 Dimethoxybenzene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.